Membrane transporters belonging to the divalent anion/Na+ symporter (DASS) family use a Na+ gradient to move Krebs cycle intermediates or sulfate across cell membranes. Since the substrates for DASS include key intermediates and/or regulators of energy metabolism, including citrate, succinate, and other C4-dicarboxylates, alterations of DASS function profoundly affect fat storage, energy expenditure, neuronal function, and life span; specific mutations in a DASS-encoded gene nearly doubled the life span of fruit flies. The group of Min Lu at Rosalind Franklin University determined the first crystal structures of substrate-bound VcINDY, a bacterial divalent DASS, and of a humanized variant, both in an inward-facing conformation. These structures reveal for the first time how a DASS recognizes two Na+ ions as well as how it distinguishes between dicarboxylate and tricarboxylate. These findings offer a solid basis for understanding how the function of DASS can be modulated for potential therapeutic intervention.
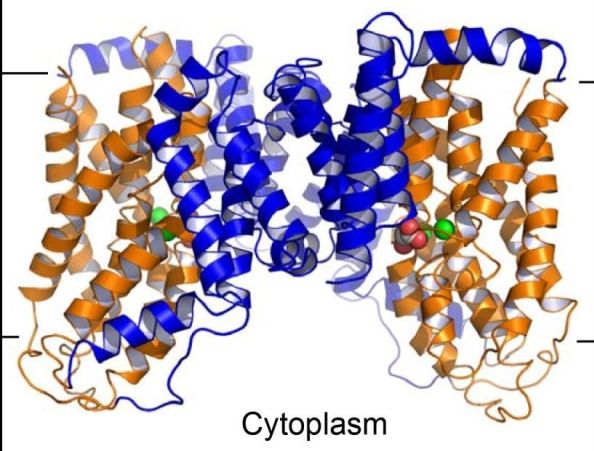 |
Figure: Structure of a DASS dimer. |
Citation: Nie, R, Stark, S, Symersky, J, Kaplan, RS, Lu, M.Structure and function of the divalent anion/Na+ symporter from Vibrio cholerae and a humanized variant, Nat. Commun. 8, 15009-1-15009-10 (2017). DOI: 10.1038/ncomms15009