TMEM175 was recently identified as a lysosomal K+ channel in eukaryotes important for setting the lysosomal membrane potential, maintaining pH stability under starved conditions, and regulating autophagosome/lysosome fusion. TMEM175 bears no sequence similarity to canonical K+ channels and also lacks the highly-conserved TVGYG signature sequence. The group of Youxing Jiang at the University of Texas Southwestern Medical Center determined the crystal structure of a prokaryotic TMEM175 homolog from Chamaesiphon minutus (CmTMEM175). The structure revealed a novel architecture for a tetrameric cation channel whose ion-selectivity mechanism is distinct from that of the classical K+ channels. Each CmTMEM175 subunit contains a six-helix-transmembrane (6-TM) domain and four subunits enclose an hour-glass-shaped ion-permeation pathway in the channel tetramer. Three layers of hydrophobic residues on the C-terminal half of each TM1 form a bottleneck along the ion conduction pathway and serve as the channel selectivity filter. Thus, the structure of CmTMEM175 reveals a hitherto unseen ion-channel mechanism for K+ selectivity.
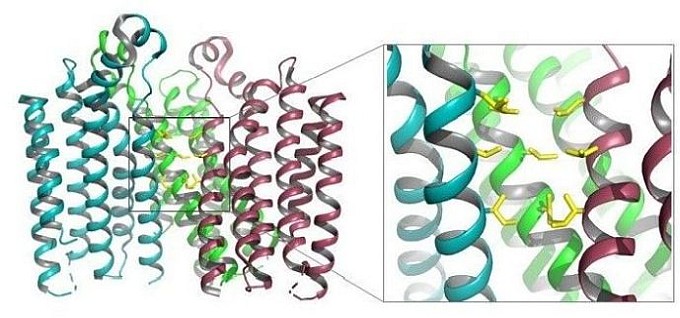 |
Figure: A cutaway of the TMEM175 tetramer, with the insert showing three layers of hydrophobic residues that form a bottleneck along the ion channel in yellow. |
Citation: Lee, C, Guo, J, Zeng, W, Kim, S, She, J, Cang, C, Ren, D, Jiang, Y. The lysosomal potassium channel TMEM175 adopts a novel tetrameric architecture, Nature 547, 472-475 (2017). DOI: 10.1038/nature23269