The Roth lab at the University of North Carolina Chapel Hill along with collaborators from UCSF determined the crystal structure of the D2 dopamine receptor in complex with the atypical antipsychotic drug Risperidone. Risperidone is a widely prescribed medication which is approved to treat schizophrenia, bipolar disorder and autism. The D2-dopamine receptor represents a principal molecular target for all approved antipsychotic drugs. Antipsychotic drug treatment is associated with many side-effects including weight gain, sedation and cardiovascular problems and these side-effects are mainly due to actions at non-D2 receptors. The structure reveals that risperidone binds in a novel way to the D2-dopamine receptor engaging both a deep binding pocket as well as a hydrophobic patch near the extracellular surface. Risperidone thus acts to stabilize the inactive state of the D2 receptor thereby inhibiting excessive dopaminergic transmission thought to occur in schizophrenia. By clarifying the mode of binding to D2 receptors, these insights should accelerate the discovery and development of safer and more effective antipsychotic drugs.
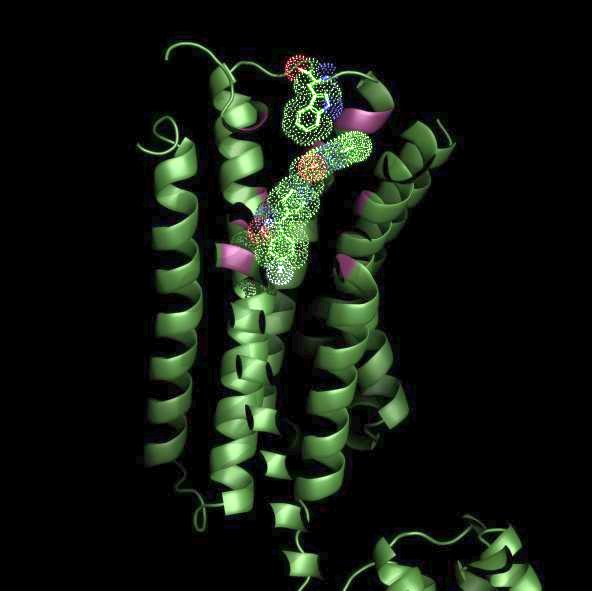 |
Figure: Risperidone bound to the D2 dopamine receptor. |
Citation: Wang, S, Che, T, Levit, A, Shoichet, BK, Wacker, D, Roth, BL., Structure of the D2 dopamine receptor bound to the atypical antipsychotic drug risperidone, Nature 555, 269-273 (2018). DOI: 10.1038/nature25758. Epub 2018 Jan 24.