The heart relies on electrical signals to contract. Mutations in genes that encode ion channels can result in severe cardiac arrhythmia, sometimes leading to sudden death. The Van Petegem lab studied the gatekeeper protein, calmodulin, which, among its many functions, regulates calcium ion channels. Calmodulin switches off calcium channels after calcium ions have entered the cytosol. This calcium-dependent inactivation process is critical for maintaining the proper cardiac signals and contraction. Calmodulin is also the target for several mutations that cause very severe heart arrhythmias. The group studied the effect of different disease mutations on the 3D structure of calmodulin. The work led to two major surprises. First, mutations can cause substantial structural changes that alter calmodulin interaction with ion channels. Second, even though many mutations cause the same type of cardiac arrhythmia, they have different effects on the structure and ability to bind calcium. This shows that different disease mutations have very different mechanisms of action, despite targeting the same protein.
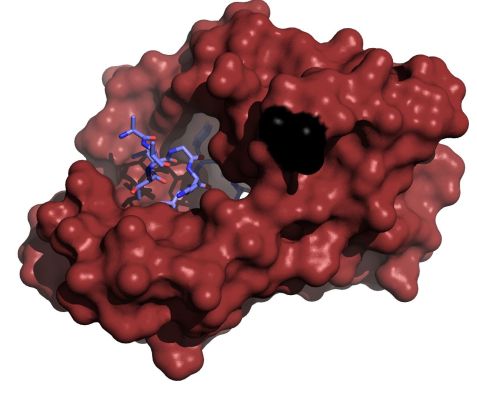 |
Figure: A disease-associated mutant calmodulin (red), where the (D95V) mutation (black) abolished calcium binding to calmodulin. EF hand 3, yet the mutated protein is still able to bind the voltage-gated calcium channel CaV1.2 by embracing the IQ domain. (purple) that is located in the C-terminal tail of the ion channel. |
Citation: Wang, K, Holt, C, Lu, J, Brohus, M, Larsen, KT, Overgaard, MT, Wimmer, R, Van Petegem, F., Arrhythmia mutations in calmodulin cause conformational changes that affect interactions with the cardiac voltage-gated calcium channel, Proc.Natl.Acad.Sci.USA 115, E10556-E10565 (2018). DOI: 10.1073/pnas.1808733115.