The groups of Robert Lefkowitz at Duke University and Andrew Kruse at Harvard Medical School determined the structure of the human angiotensin II type 1 receptor (AT1R) in an activated state. The AT1R is a critical regulator of blood pressure and kidney function in humans, and it is the target of widely used blood pressure medications called angiotensin receptor blockers or ARBs. The new structure reveals the molecular basis for AT1R activation by a peptide agonist, showing how these activators trigger a conformational change in the receptor that ultimately results in increased blood pressure. These data will help advance understanding of the molecular basis for cardiovascular function, and transmembrane receptor signaling in general.
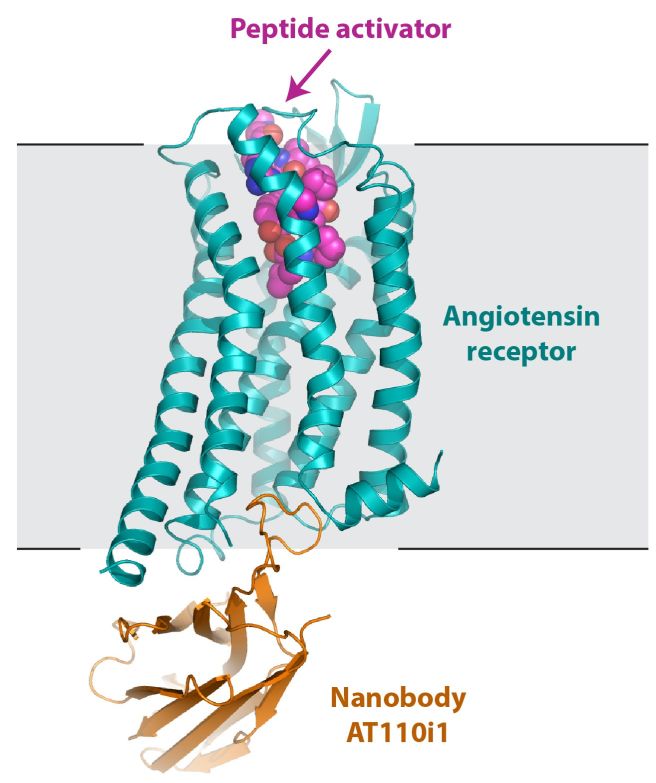 |
Figure: The structure of AT1R (blue) is shown in a schematic membrane, with an angiotensin II analog (pink) and a synthetic nanobody from a synthetic yeast-display library (brown) needed to aid crystallization. |
Citation: Wingler, LM, McMahon, C, Staus, DP, Lefkowitz, RJ, Kruse, AC., Distinctive activation mechanism for angiotensin receptor revealed by a synthetic nanobody, Cell 176, 479-490 (2019). DOI: 10.1016/j.cell.2018.12.006