 |
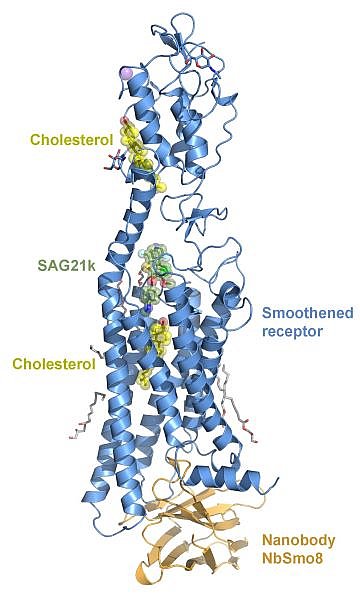
Figure: The crystal structure of Smoothened (blue) bound to a synthetic nanobody from a yeast-display library (brown) needed to aid crystallization. Also bound to Smoothened are two cholesterol molecules (yellow) and SAG21k, synthetic agonist (green). |
The group of Aashish Manglik at the University of California, San Francisco determined the crystal structure of the Smoothened receptor in an activated state. Smoothened transduces the Hedgehog signal across the cell membrane to regulate development in animals. Smoothened is also an oncoprotein and is the target of three FDA-approved drugs used to treat several cancers. Despite playing key roles in human physiology, the mechanistic basis of Smoothened activation remained a long-standing mystery. This structure revealed that cholesterol binds the transmembrane pocket of Smoothened and directly stabilizes an activated conformation of the receptor. Additional data confirmed that cholesterol binding is critical for Smoothened activation. This work suggests that new inhibitors targeting this cholesterol site may overcome cancer resistance faced by current clinical Smoothened inhibitors.
Citation: Deshpande, I, Liang, J, Hedeen, D, Roberts, KJ, Zhang, Y, Ha, B, Latorraca, NR, Faust, B, Dror, RO, Beachy, PA, Myers, BR, Manglik, A, Smoothened stimulation by membrane sterols drives Hedgehog pathway activity, Nature 571, 284-288 (2019). DOI: 10.1038/s41586-019-1355-4