 |
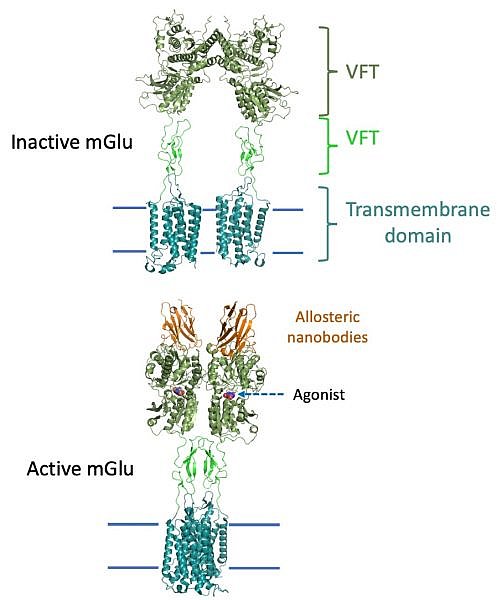
Figure: Structures of inactive and active mGlu, where the CRD are shown in lime green. |
The groups of Brian Kobilka, Georgios Skiniotis and collaborators used a combination of crystallography and cryo-electron microscopy to determine the inactive- and active-state structures of a metabotropic glutamate receptor (mGlu). mGlus play important roles in regulating neuronal responses to the neurotransmitter glutamate. The mGlus belong to the Family C G protein coupled receptor (GPCRs), which are distinguished from other GPCR families in that they are obligate dimers that possess a large amino-terminal extracellular agonist binding domain - the Venus flytrap (VFT) domain. The VFT domains are linked to the G protein coupling transmembrane domains by cysteine rich domains (CRD). Their structures show that agonist binding at the VFTs leads to a compaction of the intersubunit dimer interface, thereby bringing the CRDs into close proximity. Interactions between the CRDs and the second extracellular loops of the receptor enable the rigid body repositioning of the transmembran domains, which come into contact with each other to initiate signaling. This structure was facilitated by an allosteric nanobody that stabilized the VFT domain in the active state.
Citation: Koehl, A, Hu, H, Feng, D, Sun, B, Zhang, Y, Robertson, MJ, Chu, M, Kobilka, TS, Laeremans, T, Steyaert, J, Tarrasch, J, Dutta, S, Fonseca, R, Weis, WI, Mathiesen, JM, Skiniotis, G, Kobilka, BK, Structural insights into the activation of metabotropic glutamate receptors, Nature 566, 79-84 (2019). DOI: 10.1038/s41586-019-0881-4