When heart muscles contract, calcium ions are released from the intracellular stores in the sarcoplasmic reticulum through the specialized, 2.2-MDa ion channel known as the Ryanodine receptor (RyR). This channel of key importance in muscle contraction is the site of many mutations associated with arrhythmia, muscle weakness, and a dangerous reaction to certain anesthetics. In the heart, the RyR is under control of stress signals. For example, activation of beta-adrenergic receptors by adrenalin leads to activation of kinases (PKA, CaMKII) that can phosphorylate the RyR and facilitate channel opening and Ca2+ release. In normal conditions, this supplies the increased demand for Ca2+ during exercise or stress, but excessive phosphorylation of RyRs contributes to heart rhythm disorders, muscle fatigue, etc. The Van Petegem lab determined high-resolution crystal structures of PKA bound to its interaction domain in the RyR, which revealed the major surprise of a larger than expected interface where a long loop of the RyR forms a 'lasso' around a PKA subdomain. The interface is the site of many mutations that cause CPVT, a severe cardiac arrhythmia. In addition, the team discovered that RyR phosphorylation by CaMKII increases PKA efficiency. High-resolution structures show that the phosphorylation results in formation of a new alpha-helix, which in turn increases the affinity of PKA for the RyR.
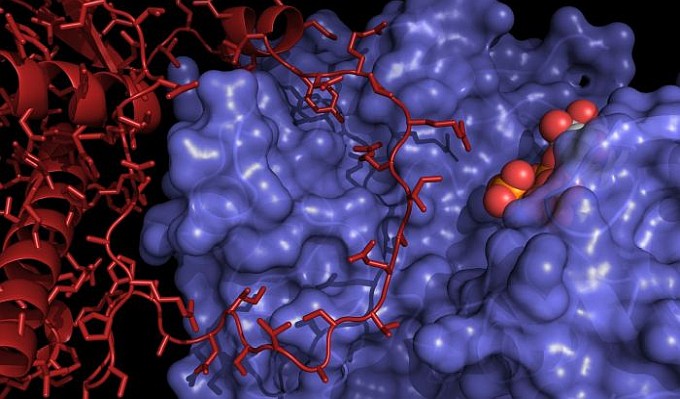 |
Figure: Structure of the PKA catalytic subunit (blue surface) bound to the phosphorylation domain of RyR2, the cardiac RyR (red). A co-crystallized ATP analog is shown in spheres. |
Citation: Haji-Ghassemi, O, Yuchi, Z, Van Petegem, F., The cardiac Ryanodine receptor phosphorylation hot spot embraces PKA in a phosphorylation-dependent manner, Molec. Cell 75, 1-14 (2019). DOI: 10.1016/j.molcel.2019.04.019.