The groups of Robert Lefkowitz at Duke University and Andrew Kruse at Harvard Medical School determined crystal structures of the human angiotensin II type I receptor (AT1R), a G-protein coupled receptor (GPCR) that controls cardiovascular and kidney function, in complex with its endogenous activating hormone angiotensin II (AngII) and two β-arrestin biased activators. When a GPCR is activated, cellular signaling is propagated through both G-proteins and β-arrestins. Some ligands preferentially or exclusively activate one pathway, which is known as "biased signaling". Since G-protein signaling and β-arrestin signaling mediate distinct physiological responses, GPCR drugs that promote biased signaling in many cases may have superior therapeutic profiles (e.g., reducing on-target side effects). In the AT1R G-protein signaling increases blood pressure, whereas β-arrestin signaling promotes cardioprotective effects. The new structures show that β-arrestin biased activators stabilize a conformational state in the base of the ligand binding site and receptor core that is distinctly different from that induced by AngII. These results provide insight into the molecular basis for biased signaling in GPCRs and may aid in the development of improved therapeutics targeting these receptors.
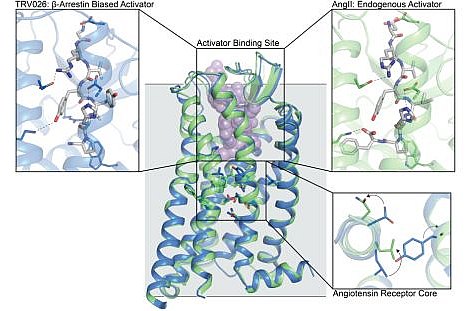 |
Figure: Crystal structures of the human angiotensin II receptor type 1 bound to the endogenous ligand angiotensin II (green) and the biased agonist TRV026 (blue) are overlaid, showing how the two ligands induce distinct structural rearrangements in the receptor core. |
Citation: Wingler, LM, Skiba, MA, McMahon, C, Staus, DP, Kleinhenz ALW, Suomivuori, CM, Latorraca, NR, Dror, R, Lefkowitz, RJ, Kruse, AC, Angiotensin and biased analogs induce structurally distinct active conformations within a GPCR, Science 367, 888-892 (2020). DOI: 10.1126/science.aay9813