The group of Demet Araç and collaborators determined the x-ray crystal structure of the full-length extracellular region of Gpr126, an adhesion G-protein coupled receptor with essential roles in axon myelination, ear development, and heart development. The structure revealed an interesting architecture of this region that is important for its function, and they also found that different forms of Gpr126 can adopt different architectures to possibly carry out different functions. The structure also helped in identifying a key functional site on the protein, which led to many defects when mutated in zebrafish. These results demonstrate that Gpr126 utilizes a multi-faceted dynamic approach to regulate receptor function and provide relevant insights for drug design against Gpr126 and other adhesion G-protein coupled receptors.
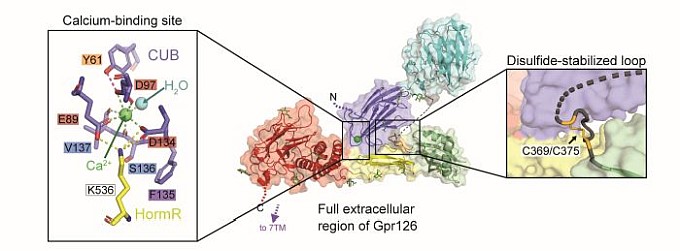 |
Figure: (center) Crystal structure of the full extracellular region of zebrafish Gpr126, colored by domain (dark blue: CUB, cyan: PTX, green: SEA, yellow: HormR, red: GAIN). (left) Conserved calcium-binding site within CUB domain mediates interdomain interactions and is an important functional site. (right) Conserved disulfide-stabilized loop contributes to the stabilization of the closed conformation. |
Citation: Leon, K, Cunningham, RL, Riback, JA, Feldman, E, Li, J, Sosnick, TR, Zhao, M, Monk, KR, Araç, D., Structural basis for adhesion G protein-coupled receptor Gpr126 function, Nat.Commun. 11:194 (2020). DOI: 10.1038/s41467-019-14040-1