Ian Wilson's lab at The Scripps Research Institute reports the structure of the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein with a bound Fab fragment of the human neutralizing antibody CR3022. CR3022, isolated more than 15 years ago from a convalescent SARS patient, targets a highly conserved epitope on the RBD.
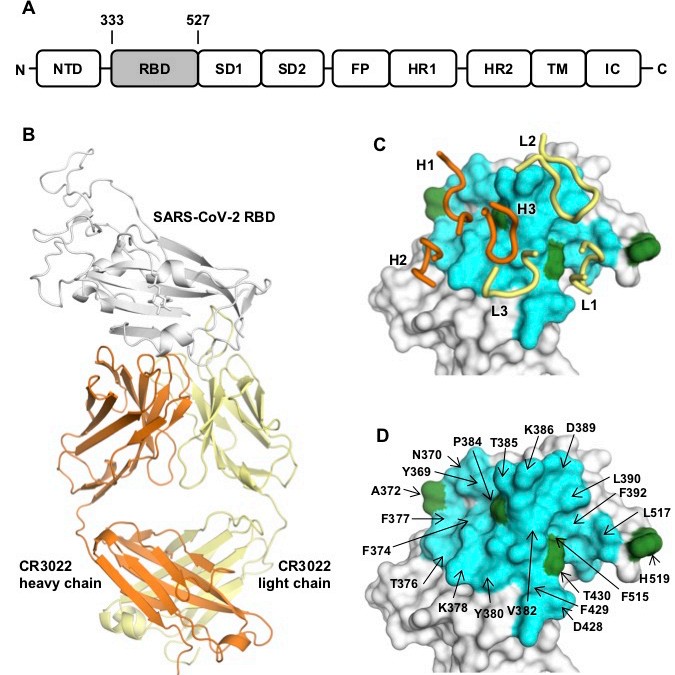 |
Figure: Crystal structure of CR3022 in complex with SARS-CoV-2 RBD. (A) Overall topology of the SARS-CoV-2 spike glycoprotein. NTD: N-terminal domain, RBD: receptor-binding domain, SD1: subdomain 1, SD2: subdomain 2, FP: fusion peptide, HR1: heptad repeat 1, HR2: heptad repeat 2, TM: transmembrane region, IC: intracellular domain. (B) Structure of CR3022 Fab in complex with SARS-CoV-2 RBD. CR3022 heavy chain is colored in orange, CR3022 light chain in yellow, and SARS-CoV-2 RBD in light grey. (C-D) Epitoperesidues on SARS-CoV-2 are colored in cyan and green. CDR loops are labeled. |
Citation: M. Yuan, N.C. Wu, X. Zhu, C.D. Lee, R.T.Y. So, H. Lv, C.K.P. Mok, I.A. Wilson, Science, 03 April 2020, DOI: 10.1126/science.abb7269.
See also the Scripps Institute News Report.