Adhesion G protein-coupled receptors (aGPCRs) are important cell-surface proteins involved in various biological processes like brain development and organ formation. Understanding and targeting the specific functions of these receptors without affecting others has been challenging due to their complex structure and diverse roles. A recent study from the group of Demet Araç and collaborators at The University of Chicago, has made a breakthrough by engineering a specialized synthetic antibody-like binder called LK30, which targets a specific aGPCR known as Latrophilin-3/ADGRL3. Remarkably, LK30 only activates the downstream signaling of ADGRL3 and not its closely related form, ADGRL1. Additionally, by targeting a specific domain on ADGRL3's surface, LK30 disrupts its interaction with one of its binding partners called teneurin, while leaving its interaction with another protein, FLRT3, unaffected. This discovery opens up new possibilities for finely tuning the activities of aGPCRs, which could have significant implications for various diseases and biological processes. The study by Kordon et al. demonstrates the potential of using tailored antibodies to precisely modulate the functions of specific aGPCR isoforms and their interactions with different ligands. Such targeted approaches could lead to the development of more effective therapies for conditions ranging from neurodevelopmental disorders to cancers.
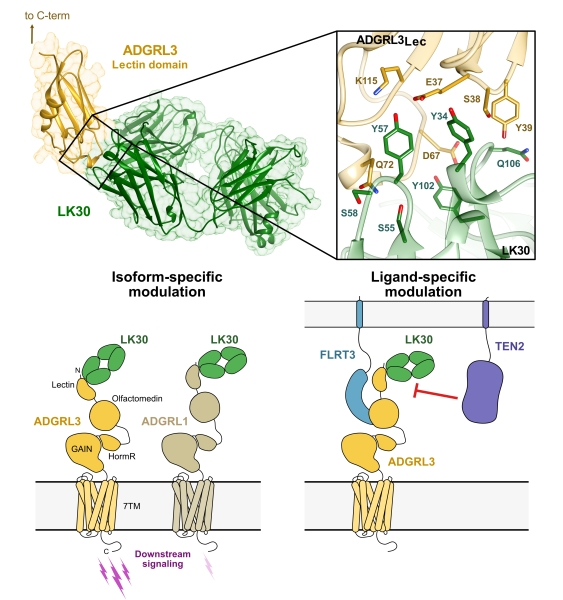 |
Figure: (Top) The crystal structure of the ADGRL3 lectin domain (yellow) in complex with LK30 (green) with a close-up view of the ADGRL3/LK30 interface. (Bottom left) Binding of LK30 to ADGRLs modulates the receptor activity in an isoform-specific manner. (Bottom right) LK30 breaks the interaction of ADGRL3 with TEN2, whereas it has no effect on the interaction of ADGRL3 with FLRT3. |
Citation: Kordon, SP, Dutka, P, Adamska, JM, Bandekar, SJ, Leon, K, Erramilli, SK, Adams, B, Li, J, Kossiakoff, AA, Araç, D, "Isoform- and ligand-specific modulation of the adhesion GPCR ADGRL3/Latrophilin3 by a synthetic binder," Nat. Commun. 14, 635 (2023). DOI: 10.1038/s41467-023-36312-7