The Saphire group at the La Jolla Institute for Immunology determined a 3.1 Å structure of the Ebola virus glycoprotein (GP) in complex with Inmazeb (REGN-EB3), a three-antibody cocktail against Ebola virus. Inmazeb is the first FDA-approved therapeutic for Ebola virus disease. In a randomized, controlled trial, Inmazeb substantially increased the likelihood of survival following Ebola virus infection. The recent molecular structure includes Fab fragments of the three monoclonal antibody components, REGN3470, REGN3471 and REGN3479, that bind three non-overlapping sites on Ebola GP: the glycan cap, receptor-binding site and fusion loop, respectively. A GM/CA@APS beamline was used to solve the crystal structure of REGN3471. Importantly, the structure allowed regions of the glycan cap that were disordered in previous structures now to be modeled. Further, modeling of REGN3470 and REG3471, which have only moderate neutralization potency, showed that the immune-interacting Fc regions are more accessible in these antibodies for FcγR-IIIA receptor binding, indicating a basis for the enhanced Fc-mediated immune effector activity of these antibodies. The combination of these two, plus REGN3479, which targets a conserved and essential pieces of GP's machinery, likely afforded the high resistance of Inmazeb to escape mutations. Taken together, this structure demonstrates how targeting multiple, non-overlapping epitopes can be used to develop an effective and durable therapeutic against a lethal viral infection.
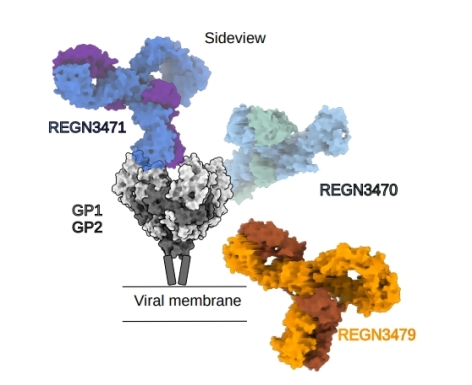 |
Figure: Structure of the Ebola virus glycoprotein (GP) in complex with Inmazeb (REGN-EB3), a three-antibody cocktail against Ebola virus, which was determined using cryo-electron microscopy and X-ray crystallography. Image credit, Vamseedhar Rayaprolu, Ph.D. |
Citation: Rayaprolu, V, Fulton, BO, Rafique, A, Arturo, E, Williams, D, Hariharan, C, Callaway, H, Parvate, A, Schendel, SL, Parekh, D, Hui, S, Shaffer, K, Pascal, KE, Wloga, E, Giordano, S, Negron, N, Ni, M, Copin, R, Atwal, GS, Franklin, M, Boytz, RM, Donahue, C, Davey, R, Baum, A, Kyratsous, CA, Saphire, EO, Structure of the Inmazeb cocktail and resistance to Ebola virus escape, Cell Host Microbe 31 (2), 260-272 (2023). DOI: 10.1016/j.chom.2023.01.002