A human protein known as ZAP (zinc-finger antiviral protein) is among the many molecular systems that protect us from viral pathogens. ZAP recognizes a short sequence motif that is present in the RNA genomes of many viruses but is depleted in our genome. When ZAP finds several of these motifs, it organizes a protein complex to destroy the RNA. In a study from the labs of Melanie Ohi and Janet Smith at the University of Michigan and Paul Bieniasz at Rockefeller University, researchers defined the minimal elements needed for ZAP-mediated RNA recognition and destruction. ZAP has no nuclease activity, so it recruits another human protein for RNA degradation, a multi-domain protein known as KHNYN. The researchers found that the NYN domain is a nuclease that cleaves single-stranded RNA, but not double-stranded RNA and not DNA, and that the nuclease lacks sequence specificity. They also investigated the KHNYN KH region, which was presumed to bind RNA and assist in nuclease activity. Using data from GM/CA beamline 23-ID-B, they solved a crystal structure for the KH region (left image) and discovered that it is a double-KH and, unlike any previously characterized KH domain, does not bind RNA. Previous work from the groups established that ZAP recognizes only one RNA motif and that ZAP binds the KHNYN C-terminal domain (CTD). In biochemical experiments in the present study, they showed that the NYN active site engages an RNA substrate on the downstream side of ZAP binding the recognition motif. This led to a model for the minimal motif-recognition and RNA destruction complex (right image). In cells, this core machinery acts only when ZAP detects multiple RNA motifs, a yet-to-be-discovered process involving additional protein partners.
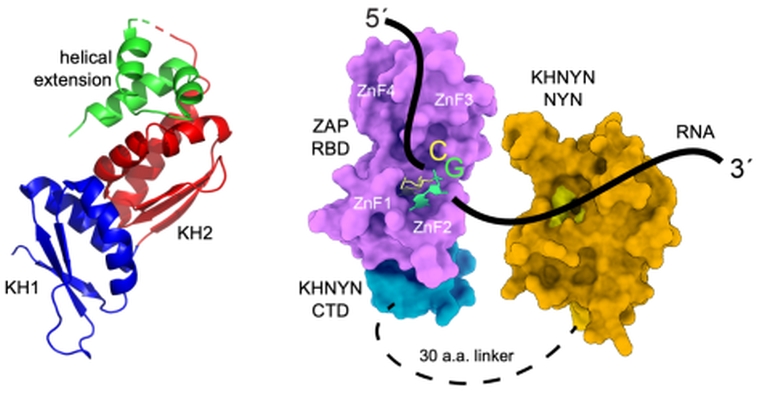 |
Figure: Human KHNYN and ZAP. Left: Structure of the KH region of KHNYN, including two KH domains in blue and red and a helical extension in green (PDB 9CS9). Right: Model of the minimal RNA destruction complex, consisting of the ZAP RNA-binding domain (pink), the KHNYN NYN nuclease domain (dark yellow with bright yellow active site), and KHNYN C-terminal domain (cyan) bound to the ZAP RBD, and RNA (black line). |
Citation: Yeoh, ZC, Meagher, JL, Kang, C-Y, Bieniasz, PD, Smith, JL, Ohi, MD, "A minimal complex of KHNYN and zinc-finger antiviral protein binds and degrades single-stranded RNA," Proc. Nat. Acad. Sci. USA 121 (52) e2415048121 (2024). doi: 10.1073/pnas.2415048121